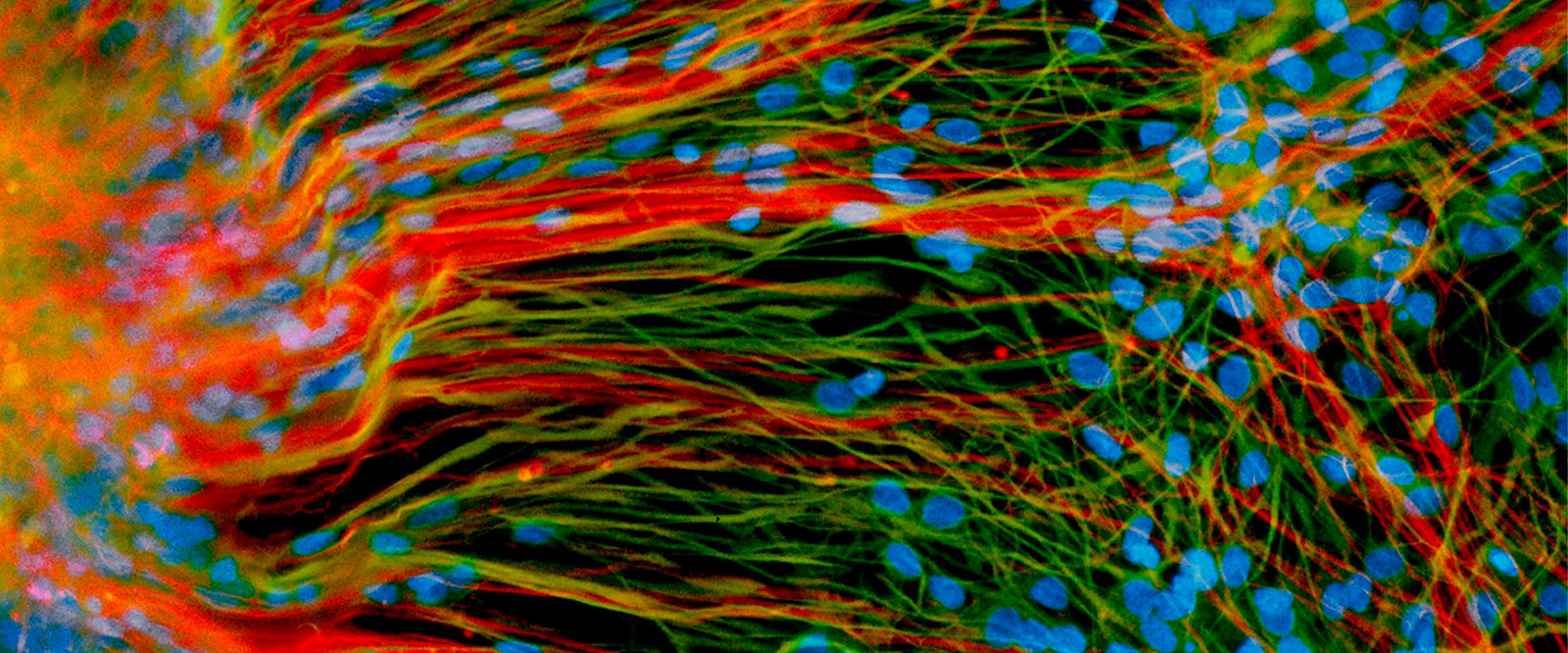
Stem Cells at 20
In the lab dish, a human embryonic stem cell can live forever. If the conditions are right, the cell will divide endlessly, providing a limitless supply of the blank-slate cells now used widely in biomedical science.
Immortality is an astonishing quality, certainly, but the feature of stem cells that has most captured the public’s imagination since they were first cultured at UW–Madison 20 years ago is the ability to manipulate them to become any of the myriad cells in the human body. The idea that specialized cells could be whipped up in large quantities to treat any number of afflictions — from dopaminergic cells for Parkinson’s to islet cells for diabetes — is a powerful one.
“For the first time, we had unlimited access to all of the basic cellular building blocks of the human body,” says James Thomson, the UW developmental biologist who first derived the original cells in 1998. “And if you make an embryonic stem cell line, that’s infinite. You can make as many cells as you want.”
But two decades on, stem cells have yet to live up to that grand clinical aspiration. Embryonic and now genetically induced stem cells from adult tissue have become lab workhorses and underpin the new field of stem cell and regenerative medicine. Worldwide, there is a score of clinical trials using stem cells, including trials for heart disease, the blinding disease macular degeneration, and spinal cord injury. And some of those trials are using the original cells Thomson made.
“I think where things are right now is pretty promising,” Thomson says. “There are a number of trials underway. Most will fail because clinical trials are hard, but some will succeed. The whole field just needs one to work.”
Stem Cells 101
Global reach
5
The number of original stem cell lines
5,200
The number of times the original five stem cell lines have been distributed around the world to:
2,350 investigators | 820 institutions | 45 countries
$1.43 billion
U.S. funding for stem cell research (1998–2017)
1,300+
U.S. scientists work with any of the original embryonic stem cell lines
UW–Madison IMPACT
284
stem cell–related patents have been issued to the Wisconsin Alumni Research Foundation (May 2018)
685
people — faculty, staff, and students — work with stem cells on the UW campus
$75M
Grants supporting stem cell projects at the UW (fiscal year 2017)
10
Wisconsin companies are devising stem cell–based products, mostly used to test drugs in lieu of using research animals
Then and now
The UW’s Thomson had high hopes for the technology in 1998. Today, he remains convinced that the legacy of stem cells will not necessarily be as therapy for replacing diseased or damaged cells, but in basic understanding of human development and — using engineered stem cells from patients — the cause of cell-based diseases, including diabetes, Parkinson’s, and ALS.
1998: Stem cell predictions
- Revolutionize basic research and understanding of human and animal development
- Use to screen drugs before using in humans
- Develop treatments — including tranplants and replacement of diseased cells and neurons — within 10 years
2018: Stem cell reality
- Use to study basic development and to model diseases in the laboratory
- Test the good and bad effects of potential new drugs on human cells, rather than in animal models
- The first clinical trials for treating condtions like spinal cord injury, eye disease, heart disease, and Parkinson’s are underway; therapeutic applications of stem cells have not yet been realized
Published in the Winter 2018 issue



Comments
Steven williamson March 12, 2022
Interesting about the life span of stem cells.