Hope for Failing Eyes
UW scientists work wonders with retinal cells grown from stem cells.
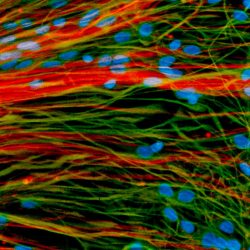
Retinal cells grown from stem cells can reach out and connect with neighbors, according to a new UW study, completing a “handshake” that may show the cells are ready for trials in humans with degenerative eye disorders.
More than a decade ago, UW–Madison researchers developed a way to grow organized clusters of cells, called organoids, that resemble the retina, the light-sensitive tissue at the back of the eye. They coaxed human skin cells reprogrammed to act as stem cells to develop into layers of several types of retinal cells that sense light and ultimately transmit what we see to the brain.
“We wanted to use the cells from those organoids as replacement parts for the same types of cells that have been lost in the course of retinal diseases,” says UW ophthalmology professor David Gamm.
Gamm and UW–Madison collaborators showed that dish-grown retinal cells called photoreceptors respond like those in a healthy retina to different wavelengths and intensities of light. Once they are separated from adjacent cells in their organoid, they can reach out toward new neighbors with biological cords called axons and create the connections called synapses that allow the cells to process sensory information.
“We’ve been quilting this story together in the lab, one piece at a time, to build confidence that we’re headed in the right direction,” says Gamm, who patented the organoids and cofounded Opsis Therapeutics, which is adapting technology based on the UW–Madison discoveries to treat human eye disorders. “It’s all leading, ultimately, to human clinical trials, which are the clear next step.”
Published in the Spring 2023 issue


Comments
Prof. Dr. M. Amjad Hossain June 18, 2024
I found the “Hope for Failing Eyes” article incredibly inspiring. As someone with a family history of vision problems, it’s heartening to learn about the advancements in eye care and treatments. The detailed explanations on emerging technologies and therapies offer hope to many facing vision loss. It’s encouraging to see the progress being made and the potential for improved quality of life for those with failing eyesight. Thank you for sharing this valuable information!